Antithrombotics in COVID-19 outpatients
A study published in JAMA investigated whether adding antithrombotic therapy to placebo reduced major cardiopulmonary complications over a 45-day treatment period in COVID-19 outpatients.
Antithrombotic therapy is not indicated in symptomatic but stable COVID-19 outpatients
A study published in JAMA investigated whether adding antithrombotic therapy to placebo reduced major cardiopulmonary complications over a 45-day treatment period in symptomatic but clinically stable COVID-19 outpatients. The results suggest that not treating these patients with anticoagulation or anticoagulant therapy may be the best course of action.
Critically ill patients with SARS-CoV-2 infection have an increased risk of arterial and venous thrombosis, which often manifests as pulmonary microvascular thrombosis that can severely compromise treatment and complicate COVID-19 pneumonia. To date, a number of randomised clinical trials have evaluated the usefulness of anticoagulant and antiplatelet interventions in hospitalised COVID-19 patients. However, the appropriateness of prescribing these drugs in clinically stable outpatient COVID-19 patients is controversial, given the absence of randomised trials in this setting. This issue is of considerable public health importance, as most individuals infected with SARS-CoV-2 do not require hospitalisation and are treated at home.
A year ago, a group of researchers decided to study whether symptomatic COVID-19 outpatients should be given anticoagulant or antiplatelet therapy to prevent thrombotic events reported among some COVID-19 patients. Data collected from a randomised, double-blind, placebo-controlled trial suggest a very low rate of thrombotic complications in the patients studied. For mildly symptomatic COVID-19 outpatients who have been ill at home for at least one week and who remain clinically stable and have no risk factors for thrombotic events, antithrombotic therapy is not warranted.
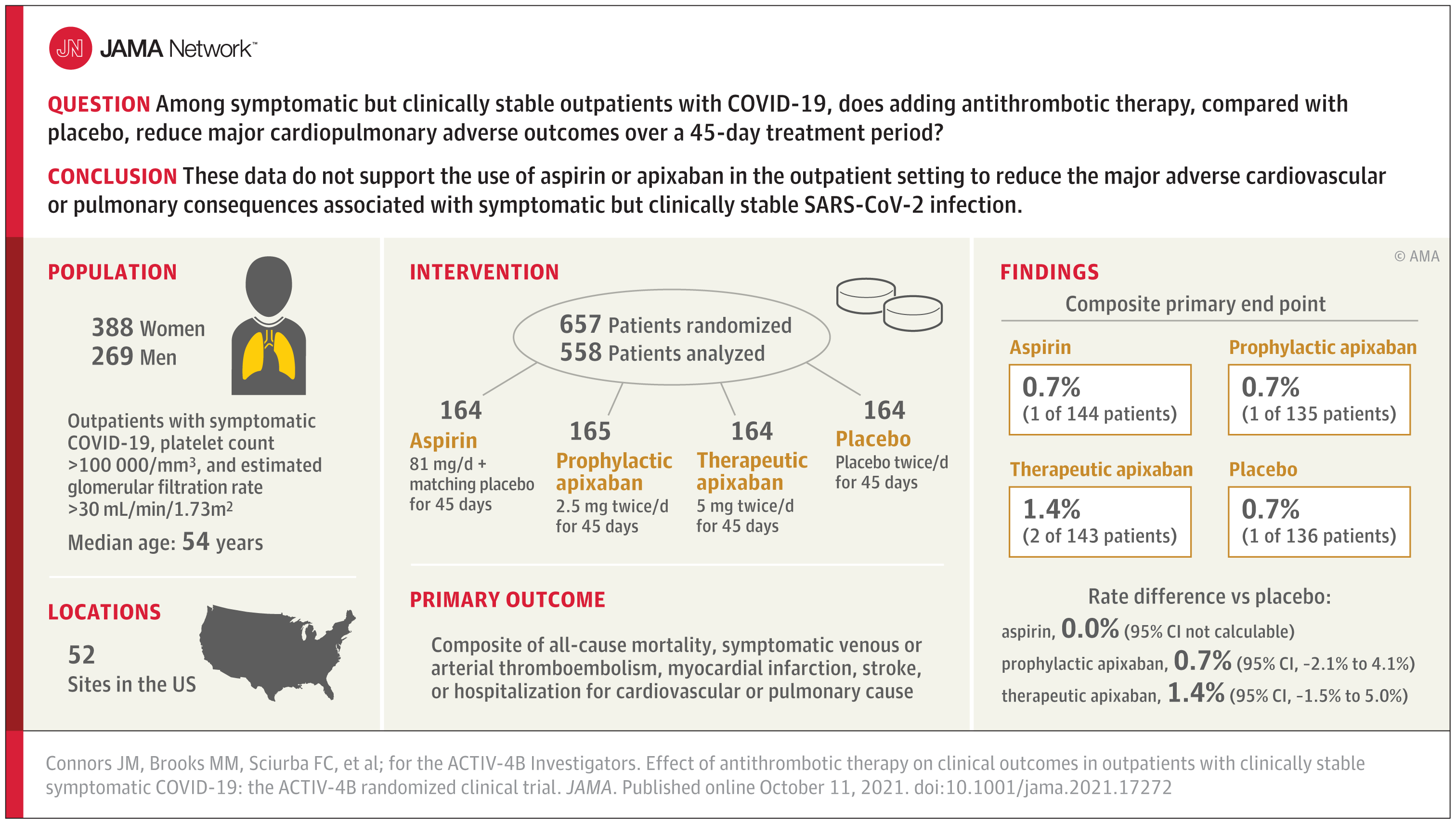
The ACTIV-4B COVID-19 Outpatient Thrombosis Prevention Trial included mildly symptomatic but clinically stable COVID-19 outpatients who were randomised into one of four arms. They were randomly assigned in a 1:1:1 ratio to aspirin (81 mg orally once daily; n = 164), apixaban at a prophylactic dose (2.5 mg orally twice daily; n = 165), apixaban at a therapeutic dose (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days. The primary efficacy outcome for the study was a composite of all-cause mortality, symptomatic venous thromboembolism, myocardial infarction, stroke, transient ischaemic attack, systemic embolism, major adverse limb events and hospitalisation for cardiovascular or pulmonary causes at 45 days. The primary safety outcome was bleeding at 45 days.
A total of 657 patients were randomised (median age, 54 years [IQR, 46-59]; 59% women). The median times from diagnosis to randomisation and from randomisation to initiation of study treatment were 7 days and 3 days, respectively. Among the 558 patients initiating treatment, the primary outcome occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5 mg apixaban group, and 1 patient (0.7%) in the placebo group.
The risk differences compared to placebo for the primary outcome were 0.0% (95% CI not calculable) in the aspirin group, 0.7% (95% CI, -2.1% to 4.1%) in the 2.5 mg apixaban group, and 1.4% (95% CI, -1.5% to 5.0%) in the 5 mg apixaban group. The differences in risk compared to placebo for bleeding events were 2.0% (95% CI, -2.7% to 6.8%), 4.5% (95% CI, -0.7% to 10.2%) and 6.9% (95% CI, 1.4% to 12.9%), respectively, among participants initiating therapy in the aspirin, prophylactic apixaban and therapeutic apixaban groups, although none were severe.
Study lead author Dr. Jean Connors, a hematologist at Brigham and Women's Hospital in Boston, said, "Physicians caring for mildly symptomatic COVID-19 outpatients ask us what the best course of treatment is for these extremely common patients. For those who are a week or more past the time of diagnosis of COVID-19, who are clinically stable and have no other risk factors, our data show that the best course of action is probably not to treat with antithrombotics unless there is no other indication for such treatment."
The study, which was stopped due to a lower-than-expected control event rate, shows that the use of ASA and NOA in symptomatic COVID-19 outpatients did not reduce hospitalisation rates compared to placebo.
What are the main implications of the ACTIV-4B Outpatient Thrombosis Prevention Trial? First, the results of the study may have direct effects on treatment decisions in clinical practice. Given the null results for major cardiovascular and pulmonary events, at present, the use of aspirin or apixaban for symptomatic but stable outpatients with COVID-19 does not seem justifiable. Second, the results of the ACTIV-4B Outpatient Thrombosis Prevention Trial provide useful indications for conducting studies on antithrombotic therapy in outpatients with COVID-19. At least 10 randomised clinical trials are ongoing in this setting. These studies are testing therapies with antiplatelet agents, oral anticoagulants and low molecular weight heparins. Most studies are open-label and sample sizes are variable. The most typical primary outcomes are the need for hospitalisation or a combination of major cardiopulmonary events. In an editorial published in JAMA, Dr. Berwanger writes that the lower-than-expected event rates observed in the ACTIV-4B Outpatient Thrombosis Prevention Trial should prompt steering committees and independent data monitoring committees of ongoing studies to review issues such as statistical power, outcome choices, recruitment feasibility and even futility.
References:
1. Connors JM, Brooks MM, Sciurba FC, et al. Effect of Antithrombotic Therapy on Clinical Outcomes in Outpatients With Clinically Stable Symptomatic COVID-19: The ACTIV-4B Randomized Clinical Trial. JAMA. Published online October 11, 2021. doi:10.1001/jama.2021.17272
2. Berwanger O. Antithrombotic Therapy for Outpatients With COVID-19: Implications for Clinical Practice and Future Research. JAMA. Published online October 11, 2021. doi:10.1001/jama.2021.17460 Press Release. NIH ACTIV-4B COVID-19 Outpatient Thrombosis Prevention Trial Ends Early. Brigham and Women's Hospital. June 22, 2021