Omeprazole: Adjuvant therapy for COVID-19?
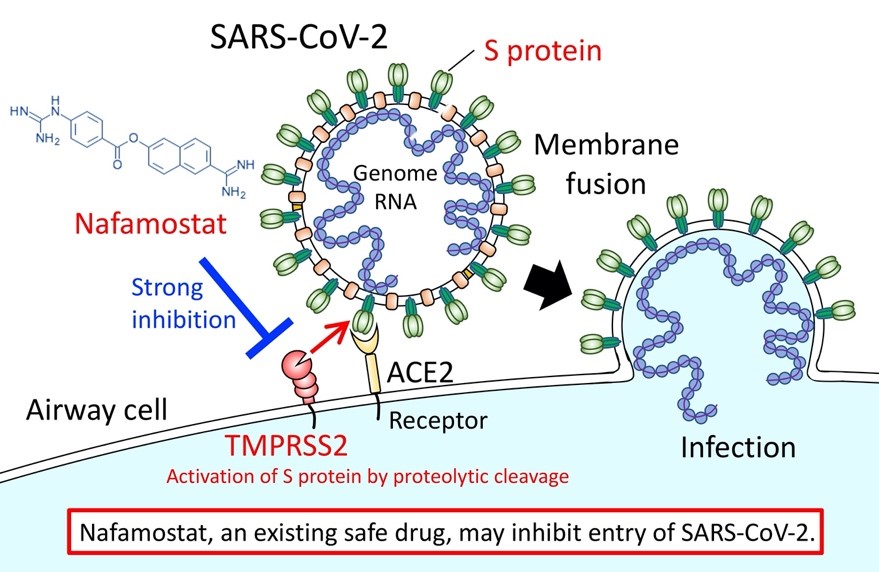
A study group compared the SARS virus and the new coronavirus responsible for COVID-19. Some differences are highlighted in the interaction of viruses with the ACE2 receptor and the enzyme TMPRSS2.
Preliminary research identifies potential drug combinations for the treatment of COVID-19
A study group compared the SARS virus and the new coronavirus responsible for COVID-19. In the "preprint" article, some differences are highlighted in the interaction of viruses with the ACE2 receptor and the enzyme TMPRSS2. The research also examines some drugs that could be candidates for COVID-19 therapy such as camostat, nafamostat, aprotinin, and omeprazole.
Made in cooperation with our partners from esanum.fr and esanum.it
A team of German and British researchers analyzed the new coronavirus. The work, not yet reviewed (the preprint has not been certified by peer review), identified substantial differences between the two viruses (SARS and the novel coronavirus). They differ in the type of cells they can infect and their drug sensitivity profiles. Drugs active against the SARS-CoV virus are not always effective against SARS-CoV-2. According to the authors, these two closely related viruses still seem to differ in many respects.
The interaction with human ACE2 receptors varies between the SARS virus and the new coronavirus. The research group examined various human cell lines and their susceptibility to viral infection and found that although the presence of the ACE2 receptor is important, it is not the only factor involved in the infection. Using bioengineering techniques, they found that SARS-CoV-2 infection is not only directly related to increased levels of ACE2.
They also examined the enzyme TMPRSS2, which is involved in the infection process. This enzyme is a target of Camostat, a serine protease inhibitor, also known as serpin, and is used in the treatment of certain forms of cancer. Susceptibility to SARS-CoV-2 is also not only related to TMPRSS2 levels, as with ACE2. The paper continues by looking at camostat and another similar drug, Nafamostat. In cell culture, both drugs are more active against SARS-CoV-2 than against the SARS virus. Unfortunately, the concentrations required in the cell test are still higher than these drugs seem to achieve in vivo in plasma concentrations. So we need to wait for further research to see if there really is any efficacy.
Credits: The University of Tokyo
The team also examined aprotinin, a protease inhibitor of bovine origin approved by the FDA in 1993 because it reduces blood loss and blood transfusion requirements during cardiac surgery, but its use has experienced ups and downs. Aprotinin was in fact temporarily taken off the market in 2007 due to a possible association with clotting problems, but this suspension was lifted by the EMA in 2012 after a further review of the data. In this work, aprotinin has been shown to be effective in cellular assays and has done so at concentrations below human blood therapeutic concentration levels. According to the authors, it shows more pronounced direct antiviral effects than Camostat and Nafamostat and appears to have greater potential for the treatment of individuals with SARS-CoV-2 infection.
The paper then examines another drug, omeprazole, the well-known proton pump inhibitor. In the past it has been reported to have some antiviral activity, increasing the pH in lysosomal compartments. The research team states that in their tests it interfered with the viral infection, but at levels too high for a realistic human dosage. However, the researchers report an interesting fact: simultaneous treatment with omeprazole (at human therapeutic concentrations) increased aprotinin activity by 2.7 times and increased remdesivir activity by 10 times.
According to the authors, the use of this widely used and well-tolerated drug could be studied to better assess the effectiveness of remdesivir in COVID-19 patients. The same group had noted in 2019 that omeprazole had increased the activity of acyclovir against the herpes virus. The authors indicated that the interactions highlighted deserve further investigation because the effect could be more general.
Source:
SARS-CoV-2 and SARS-CoV differ in their cell tropism and drug sensitivity profiles. Denisa Bojkova, Jake E. McGreig, Katie-May McLaughlin, Stuart G. Masterson, Marek Widera, Verena Krähling, Sandra Ciesek, Mark N. Wass, Martin Michaelis, Jindrich Cinatl Jr. bioRxiv 2020.04.03.024257; DOI: https://doi.org/10.1101/2020.04.03.024257
This article is a preprint and has not been certified by peer review.