Particle therapy: High precision technology against cancer
Particle therapy fights with a prescribed high load and extreme precision against radioresistant tumors, while preserving healthy tissue.
Particle therapy fights with high loads and extreme precision against radioresistant tumors
Article translated from the original French version
There are three main types of cancer treatment that can be applied alone or in combination: surgery, chemotherapy and radiation therapy. The latter consists of using ionizing radiation (mainly X-rays or electron beams, but sometimes also internal radioactive sources) in the local or local-regional treatment of cancerous tumors. The idea is to deliver the prescribed dose to the tumor area while preserving the surrounding healthy tissue as much as possible.
However, despite major advancements in terms of beam quality and beam forming, certain types of tumors known as "radioresistant" (~2% of cases) cannot be treated by conventional radiotherapy.
Irradiating tumours with protons and carbon ions
There is an alternative and increasingly promising technique used to treat this type of radioresistant tumor. Particle therapy, also referred to as hadron therapy, consists of irradiating tumors not with X-ray beams (photons) but rather with charged particles such as protons or even carbon ions. The big difference between photons and these charged atomic nuclei is the way these two types of particles deposit their energy in the patient's body.
While proton therapy is fairly well known (there are about 100 treatment centers in the world, including three in France), carbon therapy is still a relatively unknown technology. In theory, the term “hadron therapy” encompasses these two techniques. In practice, it refers more specifically to the latter.
Carbon ion therapy: unprecedented precision
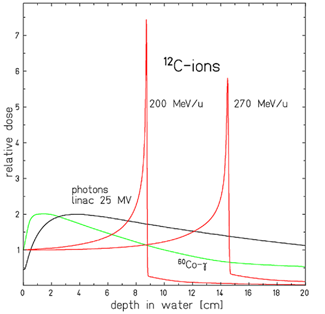
While X-ray beams deposit energy along their journey from the patient body's entrance to the exit, charged particles (protons or carbon ions) only penetrate to a certain depth and then stop. The depth of penetration varies according to the energy of the beam and the type of material it passes through.
Comparison of different dosage deposition profiles for two carbon ion beams of differing energy (in red) and two X-ray beams (in green and black). D. Schardt et al., Reviews of Modern Physics, 82 (2010), 383
Moreover, the deposition of energy from X-ray beams is continuous along a slightly decreasing profile as the depth increases (a single beam therefore deposits more energy near the surface than in the tumor, at depth). In contrast, charged particles deposit very little energy along their journey.
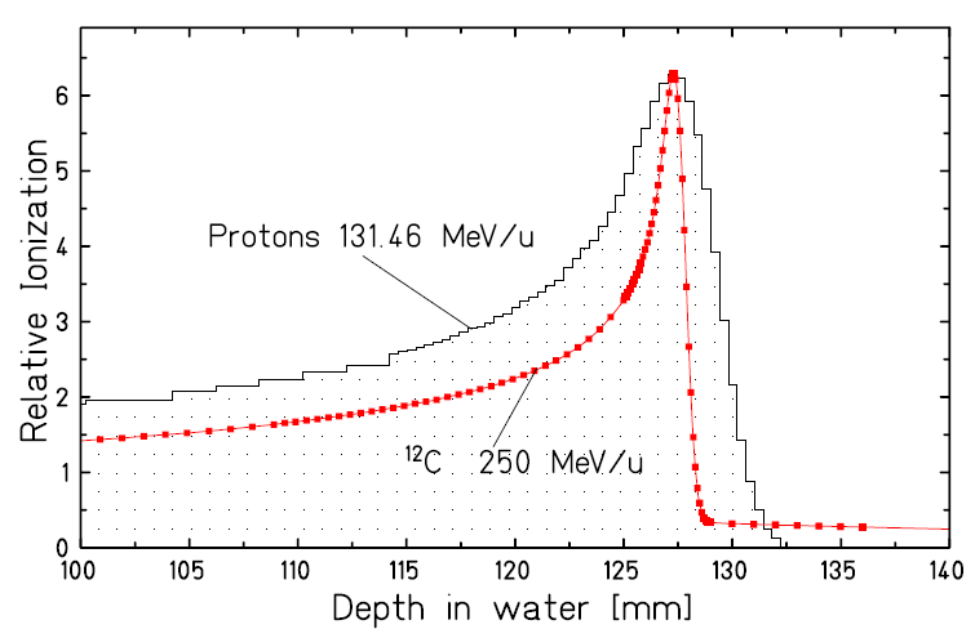
But their interaction with the material they pass through is characterized by a peak of energy deposition (called “Bragg peak”) near the place where they stop. Since the spatial and energetic dispersion of carbon ions is lower than that of protons, the Bragg peak obtained with carbon therapy is even more precise than with proton therapy, at equivalent depth.
Comparison of the Bragg peak of a carbon ion beam and a proton beam on the same journey. The Bragg peak of the proton beam is about three times wider.
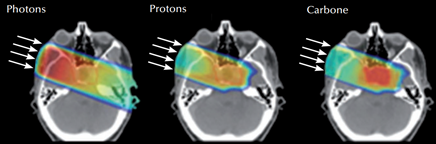
It is no surprise that interest in this type of radiation is quickly rising, as it can considerably reduce the deposition of energy in the tissue located before the tumor, almost canceling it in the tissue located after the tumor, and maximizing the deposition of the dose at the exact depth where the tumor is located.
©GCS ETOILE - Effect of a single beam of radiation in X-rays (left), proton therapy (middle) and carbon particle therapy (right). The tumor lies in the center of the skull.
The advantages of particle therapy
High effectiveness in radiation-resistant tumours
Particle therapy is particularly suitable for radioresistant tumors such as cholangiocarcinoma or pancreatic carcinoma. Paradoxically, these tumors can be very sensitive to this new type of radiation, which is then used in addition to conventional radiotherapy.
A therapy with high precision
One of its other advantages is its target accuracy. Not only does the particle beam stop in the tumor, completely avoiding the downstream tissues, it is also much thinner than an X-ray beam. This makes it necessary to move the beam during radiation treatment to irradiate the entire size of the tumor spot by spot. Such precision is invaluable when treating tumors positioned very close to radiosensitive healthy tissue, such as spinal and paraspinal tumors: sarcomas and inoperable or incompletely resected chordomas.
The effectiveness of particle therapy surpasses other treatments
In addition, the results published for carbon ion irradiation show an efficacy far above the values of non-carbon treatments. These results pertained to head and neck adenocarcinomas, mucosal melanomas, chordomas, sarcomas, hepatocarcinomas and pelvic recurrences of rectal adenocarcinomas.
A shorter therapy
Lastly, the use of carbon therapy reduces the number of sessions required. According to data from Italy, treatment with protons requires an average of 35 sessions, compared to 16 with carbon ions.
©GSI - GSI Illustration of “raster scanning” technique allowing to treat the whole tumor by varying the x, y, z position of the Bragg peak.
What are the limitations of particle therapy?
The difficulties of tumour removal
One limitation of particle therapy is its inability to follow a moving tumor in real time as its position changes with the patient's breathing (lungs, liver, pancreas, etc.). One way to solve this problem is to have the patient undergo a 4D scan, where the fourth dimension is the time of breathing. The aim is to "photograph" the oscillation of the tumor during inhaling and exhaling. During the treatment, infrared cameras follow the tumor with sensors placed on the patient and the beam is only activated when the tumor is in a position where the beam can reach it.
Logistics: the biggest limitation of particle therapy
However, the major limitation is of course a matter of logistics. Carbon ion hadrontherapy requires constructing a particle accelerator adjacent to the treatment facility. Another requirement is a moving element that can rotate around the patient without compromising accuracy at the millimeter. A facility this size is certainly a challenge, given the dimensions, the technical difficulties and the investment needed.
Cost is one of the reasons behind the small number of carbon ion therapy facilities. Currently, there are only four carbon particle therapy centers active in Europe: two in Germany (the Heidelberg Ion Beam Therapy Center, HIT, and the Marburg Particle Therapy Center, MIT), one in Austria (MedAustron in Wiener Neustadt), and one in Italy (Centro Nazionale di Adroterapia Oncologica, CNAO).
The carbon ion particle therapy in a nutshell
Carbon ion particle therapy is an innovative and highly effective technology in the treatment of radioresistant tumors that have a complex structure or are adversely positioned in the patient's body. However, it is extremely sophisticated and this is a major obstacle to its development. While Japan is a pioneer in this field with seven carbon therapy treatment centers, France is still woefully lacking.