Structure of the HCN4 pacemaker channel resolved
A recent study describes the atomic-level working mechanism of the HCN4 channel, the 'pacemaker' protein that regulates the heart's rhythmic activity.
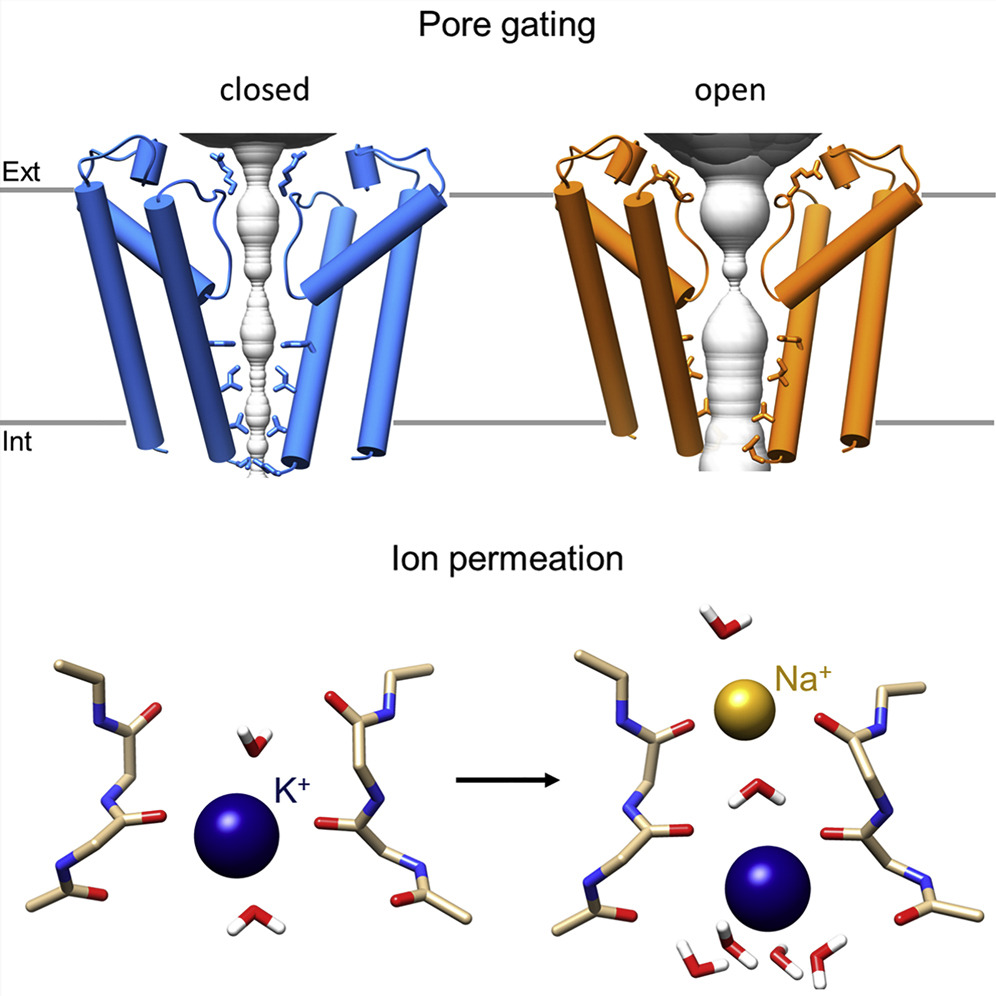
A recent study published on "Molecular Cell", describes at the atomic level the working mechanism of the HCN4 channel, the 'pacemaker' protein that regulates the rhythmic activity of the heart.
Made in cooperation with our partners from esanum.it
The heartbeat is primarily an electrical process and is by nature automatic, i.e. not dependent on external impulses. HCN channels, which are proteins that generate small electrical currents linking the end of one heartbeat to the next, contribute to this automaticity.
The international study, coordinated by the State University of Milan (Università Statale di Milano), and published in Molecula Cell, shows, for the first time, an HCN protein in an open arrangement, and makes it possible to explain how electrical charges pass through it. "The result has a strong impact in pharmacology as it provides the atomic details of ivabradine's activity, the only HCN channel inhibitor on the market, laying the groundwork for the development of new drugs," commented Andrea Saponaro, main study author.
"Ivabradine is a drug previously identified by Professors Bucchi and Di Francesco, from the Pacelab laboratory (at the univeristy's Department of Biosciences), and successfully used in the treatment of angina," explains Anna Moroni, Professor of Plant Physiology, and coordinator of the project, "but as it is not very specific for the HCN4 protein, it can lead to undesirable effects that can be addressed and resolved thanks to the knowledge provided by this study".
The work is the result of a fruitful collaboration between the Ion Channel Biophysics group of the Università Statale di Milano and Columbia University, where Anna Moroni and Andrea Saponaro have carried out research to consolidate and develop their expertise in the structural biology of membrane proteins, placing the Università Statale di Milano among the leading centres in the field.
The study benefited from the recent acquisition by the Università Statale di Milano and the CRC-Centro di Ricerca Pediatrica Romeo ed Enrica Invernizzi (Pediatric Research Center Romeo and Enrica Invernizzi) of the single-particle cryoelectronic microscopy system at the University's Department of Biosciences. Set up and led by Martino Bolognesi, Professor of Biochemistry, the cryoelectronic microscopy laboratory at the Università Statale di Milano is the first to have brought this pioneering method to Italy. Participating in the work were Paolo Swuec, now at the Human Technopole, and Antonio Chaves-Sanjuan, from the university's Department of Biosciences.
In addition to cutting-edge structural biology, a key role in understanding the mechanisms of ion permeation through the pore of HCN channels, as well as blockage by ivabradine, was played by 'in-silico' analyses such as molecular dynamics simulation, coordinated by Gerhard Thiel and Kay Hamacher, professors at the Darmstadt Polytechnic University, Germany.
The research work is part of the project coordinated by Andrea Saponaro, funded by the Cariplo Foundation, which is aimed at identifying isoform-specific characteristics of the various members of the HCN channel family. It also joins the efforts of a project funded by the Telethon Foundation, coordinated by Anna Moroni, that aims to understand and treat genetic diseases linked to HCN channels; and the project funded by the Leducq Foundation under Dario Di Francesco, professor emeritus of physiology at the university, aimed at understanding the molecular mechanisms underlying the regulation of HCN4 channel activity.
Sources:
1. Comunicato Stampa. Risolta la struttura del canale del pacemaker HCN4. Università degli Studi di Milano. 24/06/2021
2. Saponaro A, Bauer D, Giese MH, Swuec P, Porro A, Gasparri F, Sharifzadeh AS, Chaves-Sanjuan A, Alberio L, Parisi G, Cerutti G, Clarke OB, Hamacher K, Colecraft HM, Mancia F, Hendrickson WA, Siegelbaum SA, DiFrancesco D, Bolognesi M, Thiel G, Santoro B, Moroni A. Gating movements and ion permeation in HCN4 pacemaker channels. Mol Cell. 2021 Jun 18:S1097-2765(21)00446-9. doi: 10.1016/j.molcel.2021.05.033. Epub ahead of print. PMID: 34166608.