US elections: Politics, protocols, and fast-track vaccines
The SARS-CoV-2 vaccine race meets the US presidential race, turning vaccine development into a political propaganda topic and pushing pharma companies to publish ongoing trial protocols for transparency.
Modern and Pfizer publish protocols for coronavirus vaccine trials
The race for the vaccine against the SARS-CoV-2 virus meets the race for presidential elections in the United States, resulting in the vaccine development processes becoming the subject of political propaganda. Pharmaceutical companies are trying to regain the trust of the population and the scientific community by aiming for transparency and publishing the protocols of ongoing trials.
Made in cooperation with our partners from esanum.it
Modern and Pfizer, two pharmaceutical companies working on the development of a vaccine against SARS-CoV-2, have bowed to pressure from many quarters and, abandoning their traditional confidentiality, have unveiled the full protocols of their vaccine trials. They revealed details of how participants are selected and monitored, the conditions under which studies could be discontinued early in the event of problems, and the evidence that researchers will use to determine the efficacy of the vaccine. Moderna's study will involve 30,000 participants, Pfizer's 44,000.
Pharmaceutical companies usually share these documents after completion of the studies. The publication of protocols with the studies still underway (a rarity in itself) is essentially aimed at countering the growing suspicion among Americans that President Trump's calls to produce a vaccine before the November 2020 elections may lead to the release of an unsafe vaccine.
The protocol published by Moderna foresees that the confirmation of the vaccine’s effectiveness will likely occur by next year. This contrasts with the optimistic forecasts of the US president, who wants a vaccine widely available to the public as early as October 2020. Pfizer's protocol does not estimate the timing of the availability of the full results. However, its CEO has repeatedly said that the company hopes to have an answer as early as October. Moderna, on the other hand, only said that it could have the first result by the end of the year.
Moderna's forecasts reflect the conservative estimates of many researchers, including Dr. Robert R. Redfield, the director of the Centers for Disease Control and Prevention, who recently reported in the Senate that the vaccine could only be available in the second half of 2021. Hours later, President Trump contradicted him abruptly, talking about the availability of a vaccine in a few weeks.
Joseph R. Biden Jr., the Democratic presidential candidate, said that the process used to evaluate and approve a vaccine must be "totally transparent" for the community to trust. He said Trump's appeals to companies and regulators to speed up the process have shaken public confidence in vaccines and that policy should play no role in vaccine development.
Dr. Tal Zaks, health director of Moderna, the first company to publish details of its trial, said pharmaceutical companies were usually reluctant to do so for competitive reasons. "I am proud to do it," he said in an interview. "I don't think there is anything in what we are publishing that hasn't already been disclosed, but let the scientific community be the judge". Pfizer said in a statement that it does not usually release its protocols, adding: "We recognize, however, that the COVID-19 pandemic is a unique circumstance and the need for transparency is clear.”
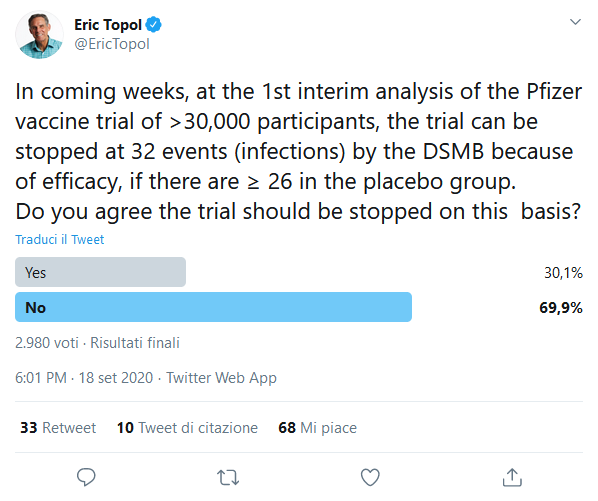
Dr. Eric Topol, a clinical trials expert at Scripps Research (San Diego, USA) gave Moderna "great congratulations" for sharing its protocol but expressed disappointment that Moderna includes people with relatively mild forms of COVID-19 in its research. He said there would be more convincing evidence of the vaccine's efficacy if the company only took moderate to severe cases into account. According to Topol, Moderna's protocol provides for the possibility of discontinuing the trial early after a relatively small number of cases, which in his view could lead to an overestimation of the vaccine's efficacy and subsequent safety problems if the vaccine were administered to millions of people.
Topol has been more critical of Pfizer's protocol because it includes even less serious cases than Moderna's and because it provides more possibilities to stop the trial early, based on a very limited number of cases. "Take your time, even extra weeks," said Dr. Topol. "No shortcuts. No one will regret it. I have been involved in clinical trials for decades. I don't know if there has ever been one more important than this. I wish it was done well and not stopped prematurely".
In the Moderna and Pfizer studies, half of the participants take the vaccine and the other half a placebo, in a double-blind arrangement. Two doses are needed, four weeks apart for Moderna and three weeks for Pfizer. Participants are then monitored to see if they develop symptoms of COVID-19 and test positive for the virus. Side effects are also monitored. Regardless of whether the vaccine is effective or not, the participants' health will be monitored for two years after the second injection.
Given the drive to bring a vaccine into the market as soon as possible, many researchers outside the trials are analyzing the details of the potential early termination of trials. If the vaccine was extremely effective, they could stop the trial because it would be unethical to continue to give some participants a placebo. The safety committee could also suspend the trial if there is evidence of harm to participants, as was recently the case in the AstraZeneca vaccine study.
The committee, called the DSMB (Data-Safety Monitoring Board), will carry out its first analysis of Moderna's efficacy data once 53 cases of COVID-19 have been diagnosed. The first analysis of Pfizer will be carried out after 32 cases. The board may recommend that Moderna's trial be discontinued after 53 cases if it is 74% effective. In the case of Pfizer, the efficacy should be approximately 77%.
The Food and Drug Administration has announced that any coronavirus vaccine must be at least 50% effective. The time needed to develop the process depends on the trajectory of the pandemic and the likelihood that participants will be exposed to the virus.
Moderna's CEO, Stéphane Bancel, said that the company will publicly report the results of the first and subsequent interim analysis. Pfizer said it will only share information about the analysis if it is decided to stop the process, either because it is very effective or because it does not seem to work.
Both Moderna and Pfizer plan to produce millions of doses of the vaccine in early 2021. But the world's population is seven billion, and everyone would need two doses. "In the first half of next year, I predict that the world will have a limited supply of vaccines, which means there will not be enough vaccines to vaccinate everyone," said Moderna's Bancel.
Sources:
1. Grady D, Thomas K. Moderna and Pfizer Reveal Secret Blueprints for Coronavirus Vaccine Trials. The New York Times. Sept 17, 2020
2. Twitter account of Dr. Eric Topol