Heterologous vaccination and Delta variant
The results of a preliminary study suggest the efficacy of heterologous anti-COVID vaccination not only for the completion of vaccination but also for a possible "third dose".
Heterologous vaccination appears to be effective against Delta variant
Results of a preliminary study published in The Lancet suggest the efficacy of heterologous COVID vaccinations not only for the completion of vaccination but also for a possible 'third dose' when humoral immunity declines.
Made in cooperation with our partners from esanum.it
Concerns about the safety profile of the COVID-19 ChAdOx1-s vaccine (AstraZeneca) led several countries to a "heterologous" approach with either BNT162B2 (Pfizer) or mRNA 1273 (Moderna) for patients who had taken the first dose, whose efficacy in determining immune response was supported by several observational studies and an RCT performed in Spain.1
This immune coverage was shown to be effective against the main variants (Alpha, Beta and Gamma), but not against the Delta variant.
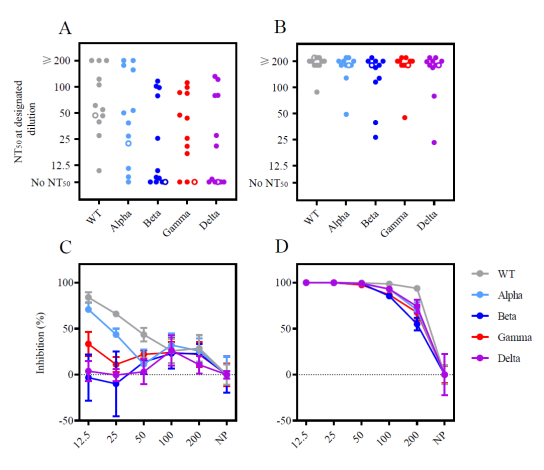
The authors of the study analysed plasma from patients who had completed the vaccination cycle with AstraZeneca and from patients who had received a heterologous vaccination and assessed its neutralising activity against SARS-CoV-2 variants, including Delta. The results show a higher absolute amount of anti-Spike IgG in the heterologous group. Furthermore, in the heterologous group the neutralisation titre at 50% (NT50) was moderate against wild type and Alpha variant virus, and further decreased for the other variants.
In contrast, all vaccines in the heterologous group achieved an NT50 of at least 25 against all variants, including Delta, and most of them (85%) achieved an NT50 >100. The amount of anti-Spike IgG correlated with high significance with the NT50 against the Delta variant in both groups.
Heterologous ChAdOx1-S/BNT162b2 vaccination induced higher levels of neutralising antibodies against Wuhan wild type (WT) virus spike protein, Alpha, Beta, Gamma and Delta than homologous vaccination. The figure is taken from the appendix to the article.
Even with all the limitations of a very small study (23 patients) that does not allow the elimination of confounders, the data of protectiveness against the Delta variant, if confirmed by other studies, are very promising in suggesting heterologous vaccination not only for the completion of vaccination but also, according to the authors, for a possible "third dose" when humoral immunity declines.
Sources & Notes:
1. Borobia AM, Carcas AJ, Pérez-Olmeda M, Castaño L, Bertran MJ, García-Pérez J, Campins M, Portolés A, González-Pérez M, García Morales MT, Arana-Arri E, Aldea M, Díez-Fuertes F, Fuentes I, Ascaso A, Lora D, Imaz-Ayo N, Barón-Mira LE, Agustí A, Pérez-Ingidua C, Gómez de la Cámara A, Arribas JR, Ochando J, Alcamí J, Belda-Iniesta C, Frías J; CombiVacS Study Group. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021 Jul 10;398(10295):121-130. doi: 10.1016/S0140-6736(21)01420-3. Epub 2021 Jun 25. Erratum in: Lancet. 2021 Aug 14;398(10300):582. PMID: 34181880; PMCID: PMC8233007.
2. Behrens GM, Cossmann A, Stankov MV, Nehlmeier I, Kempf A, Hoffmann M, Pöhlmann S. SARS-CoV-2 delta variant neutralisation after heterologous ChAdOx1-S/BNT162b2 vaccination. Lancet. 2021 Aug 17:S0140-6736(21)01891-2. doi: 10.1016/S0140-6736(21)01891-2. Epub ahead of print. PMID: 34416198; PMCID: PMC8372487.