- Clark MB, Schaefer TJ. West Nile Virus. 2023 Aug 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31334966.
- Bruno L, Nappo MA, Frontoso R, Perrotta MG, Di Lecce R, Guarnieri C, Ferrari L, Corradi A. West Nile Virus (WNV): One-Health and Eco-Health Global Risks. Vet Sci. 2025 Mar 19;12(3):288.
- Simonin Y. Circulation of West Nile Virus and Usutu Virus in Europe: Overview and Challenges. Viruses. 2024 Apr 12;16(4):599.
- Singh P, Khatib MN, Ballal S, Kaur M, Nathiya D, Sharma S, Prasad GVS, Sinha A, Gaidhane AM, Mohapatra P, Varma A, Lakhanpal S, Shabil M, Bushi G, Sah S, Abu Serhan H. West Nile Virus in a changing climate: epidemiology, pathology, advances in diagnosis and treatment, vaccine designing and control strategies, emerging public health challenges - a comprehensive review. Emerg Microbes Infect. 2025 Dec;14(1):2437244.
- Erazo D, Grant L, Ghisbain G, Marini G, Colón-González FJ, Wint W, Rizzoli A, Van Bortel W, Vogels CBF, Grubaugh ND, Mengel M, Frieler K, Thiery W, Dellicour S. Contribution of climate change to the spatial expansion of West Nile virus in Europe. Nat Commun. 2024 Feb 8;15(1):1196.
- European Centre for Disease Prevention and Control. West Nile virus infection. https://www.ecdc.europa.eu/en/west-nile-virus-infection
- European Centre for Disease Prevention and Control. West Nile virus infection - Surveillance data. https://www.ecdc.europa.eu/en/west-nile-fever/surveillance-and-disease-data
- European Climate and Health Observatory. Vector-borne diseases and climate change in Europe. 2024. https://climate-adapt.eea.europa.eu
West Nile: an exotic name, a common infection
Although often asymptomatic, West Nile virus infection can lead to devastating neurological consequences. Due to climate change, the virus will increasingly be talked about in Europe.
How is the West Nile virus transmitted?
West Nile virus is a mosquito-borne, single-stranded RNA virus belonging to the genus Flavivirus, family Flaviviridae. Birds are the primary reservoir hosts, and mosquitoes, particularly Culex species, serve as vectors. Humans and other mammals are incidental hosts, meaning they do not contribute to the transmission cycle due to low and transient viremia. The virus is transmitted mainly via mosquito bites, although rare cases of transmission through blood transfusion, organ transplantation, breast milk, and transplacental spread have been documented.
In Europe, outbreaks tend to occur between late summer and early autumn, coinciding with peak mosquito activity. In warmer regions, however, transmission may extend over longer periods. Surveillance has revealed the cocirculation of multiple lineages of WNV, with lineages 1 and 2 being the most clinically relevant.
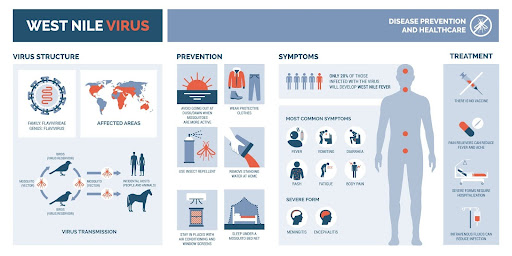
Infographic on West Nile virus (photo credit: elenabsl, Adobestock)
Symptoms of West Nile virus infection
WNV infection is asymptomatic in approximately 80% of individuals. When symptoms occur, they typically begin 3 to 14 days after a mosquito bite. The most common presentation is West Nile fever (WNF), a self-limiting, flu-like illness with abrupt onset of fever, fatigue, myalgia, headache, and sometimes gastrointestinal symptoms or a transient maculopapular rash. This mild febrile illness lasts around 3 to 6 days and resolves without sequelae.
However, in less than 1% of cases, particularly in older adults and immunocompromised patients, the virus may invade the central nervous system (CNS), causing West Nile neuroinvasive disease (WNND). Clinical manifestations include meningitis, encephalitis, or acute flaccid paralysis. Neurological symptoms may develop abruptly and progress quickly, with high risk of long-term deficits or death.
Patients with WNND may present with altered mental status, focal neurological signs, severe muscle weakness, seizures, or coma. Acute flaccid paralysis typically involves asymmetric limb weakness without sensory loss and resembles poliomyelitis. Encephalitis may include tremors, parkinsonian features, or cranial nerve palsies. Meningitis, when present, can resemble viral forms caused by enteroviruses or herpesviruses, with neck stiffness and photophobia.
Systemic complications can include myocarditis, pancreatitis, hepatitis, rhabdomyolysis, and, rarely, central diabetes insipidus. Ocular manifestations such as chorioretinitis and vitritis may occur, especially in severe cases.
West Nile: a difficult diagnosis
The diagnosis of WNV infection can be challenging, especially during the early phase or in the absence of neurological signs. Routine blood tests are non-specific and may show leukocytosis, lymphopenia, thrombocytopenia, or mild transaminase elevation. In WNND, lumbar puncture typically reveals lymphocytic pleocytosis with elevated protein and normal glucose. Early in the disease course, neutrophilic predominance may be observed.
The gold standard for diagnosis remains serology. Detection of WNV-specific IgM in serum or CSF is diagnostic; however, IgM may persist for months, and cross-reactivity with other flaviviruses (e.g., Usutu, TBEV, dengue, Zika) can complicate interpretation. A negative IgM result in the first week does not rule out infection, and repeat testing after 7-10 days may be required.
WNV-specific IgG indicates past exposure but is not diagnostic unless a four-fold rise is demonstrated in paired samples. Molecular detection via RT-PCR is most sensitive early in infection, particularly in whole blood, which may yield positive results for up to three weeks. CSF and urine may also be used but with lower sensitivity. Viral culture is rarely performed due to biosafety concerns and low sensitivity.
Differentiating WNV infection from other flavivirus infections can be particularly difficult in vaccinated individuals or those with prior exposure to related viruses. In such cases, plaque-reduction neutralization tests (PRNT) may be helpful to confirm the diagnosis.
Differential diagnosis and red flags
The clinical presentation of WNV overlaps with several infectious and non-infectious conditions. Key differential diagnoses include:
- Bacterial meningitis (e.g., Neisseria meningitidis, Streptococcus pneumoniae)
- Viral meningoencephalitis (e.g., HSV-1, VZV, enteroviruses, Listeria in elderly)
- Other arboviral encephalitides (e.g., St. Louis encephalitis, Japanese encephalitis)
- Autoimmune or paraneoplastic encephalitis
- Tick-borne diseases (Lyme neuroborreliosis, ehrlichiosis, anaplasmosis)
- Guillain-Barré syndrome and other peripheral neuropathies
In endemic areas or during the summer and early autumn months, clinicians should have a low threshold for considering WNV, especially in elderly patients presenting with fever and neurological signs. Recent travel history, exposure to mosquitoes, or blood donation should be explored.
There is no specific treatment for West Nile virus infection
There is currently no approved antiviral treatment for WNV infection. Management is primarily supportive, including hydration, analgesia, antipyretics, and in severe cases, intensive care with respiratory support. Immunoglobulin, interferon-alpha, and ribavirin have been tested in small studies but lack robust efficacy data.
Patients with WNND often require prolonged rehabilitation. Outcomes vary: while most patients with WNF recover fully, up to 50% of WNND survivors may experience long-term sequelae such as motor deficits, cognitive impairment, or chronic fatigue. The estimated mortality rate in neuroinvasive cases ranges from 5 to 10%, increasing with age and comorbidities.
The prognosis is excellent in non-neuroinvasive cases. However, even mild infections can result in lingering fatigue or depression. Therefore, follow-up should include evaluation for physical and cognitive recovery, especially in elderly or high-risk patients.
Surveillance and current trends in Europe
The European Centre for Disease Prevention and Control (ECDC) monitors WNV transmission across the continent. In 2024, over 1.100 confirmed human cases were reported in 19 EU/EEA countries. Greece and Italy remained hotspots, but sporadic cases occurred further north, including in Germany.
Germany has documented WNV circulation in birds and horses since 2018, with confirmed avian infections in 2023 and early 2024. To date, no autochthonous human cases have been reported in 2025, but public health authorities maintain active surveillance in high-risk areas. Regional monitoring includes testing of sentinel birds, horses, and mosquito pools.
Climate change and the northward shift
The expanding geographical range of WNV in Europe is closely linked to climate change. Warmer temperatures and altered precipitation patterns favor mosquito breeding and virus replication. Extended warm seasons facilitate more transmission cycles and increase the risk of human infection.
Modeling studies suggest that WNV outbreaks may become more frequent and widespread in central and northern Europe, including parts of Germany, France, and the Netherlands. Projections from the European Climate and Health Observatory indicate that by 2040–2060, WNV incidence may increase by fivefold in certain regions.
Public health preparedness must include vector control, animal surveillance, community education, and physician awareness. Integrating a One Health approach, addressing human, animal, and environmental health together, will be essential in adapting to these changes.